Abstracts
Vol. 2 No. s1 (2025): 48th National Conference of the Italian Association for the Study of Pain
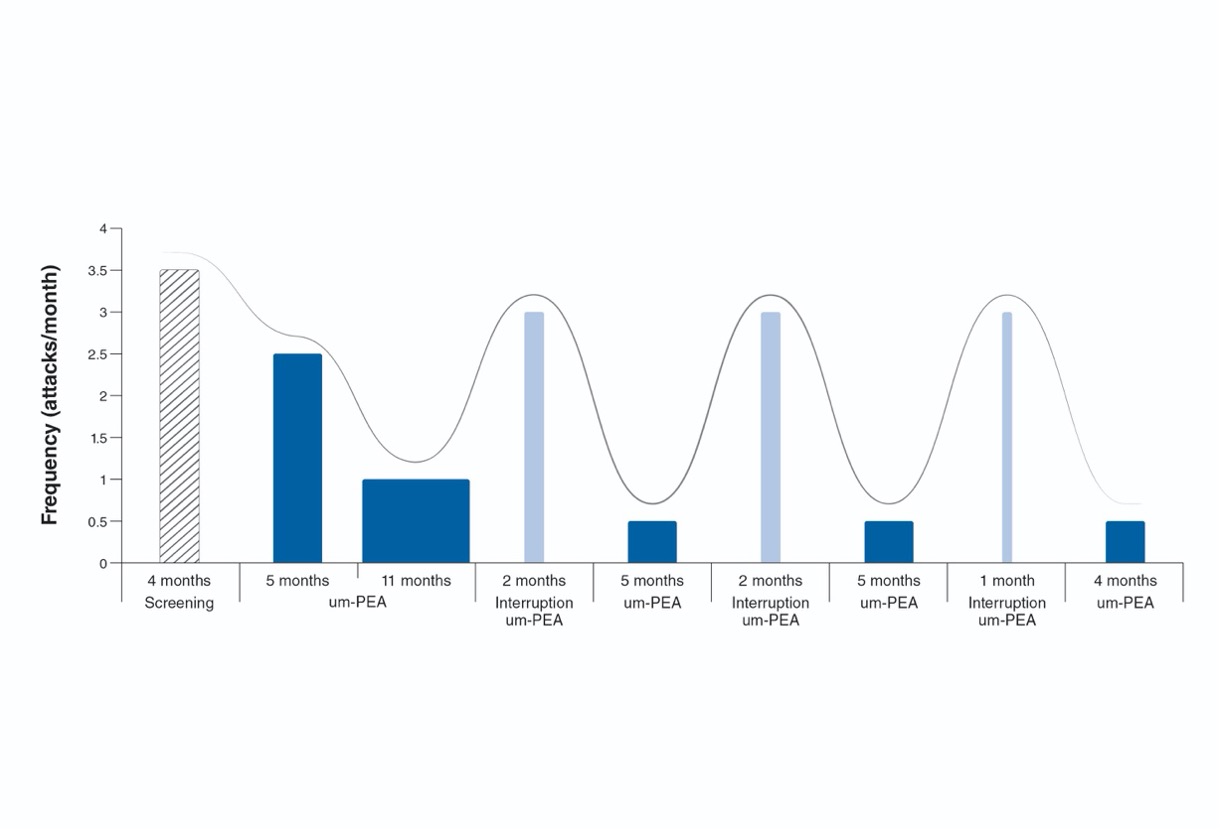
ULTRA-MICRONIZED PALMITOYLETHANOLAMIDE IN THE MANAGEMENT OF SEVERE HEADACHE: THE COMPLEX CASE OF A 13-YEAR-OLD GIRL WITH MULTIPLE NEURODEVELOPMENTAL DISORDERS
I. Turrini1, C. Monaco1, I. Contaldo1, C. Veredice1, C. Brogna1, D. Rigante2 | 1Pediatric Neurology Unit, Policlinico Universitario A. Gemelli IRCCS, Roma; 2Department of Life Sciences and Public Health, Policlinico Universitario A. Gemelli IRCCS, Roma
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 September 2025
23
Views
0
Downloads